[FDA's] Division of Drug Marketing, Advertising & Communication (DDMAC) has added a second DTC review group to meet soaring demand for advertising approvals, said DTC group leader Melissa Moncavage."The number of DTC ads submitted is expected to climb again for 2006," reports MM&M. You think?
DDMAC fielded 9,285 consumer promotions in 2005 --– up from 8,417 in 2004 and 6,099 in 2003. The number of DTC ads submitted is expected to climb again for 2006.
I plotted these numbers in the chart below and added an estimate for 2006, noting that PhRMA's Guiding Principles for DTC, which call for submission of all DTC ads to the FDA for approval before release, became effective January 1, 2006.
PhRMA's principles are voluntary, but in 2005, Astrazeneca proposed a mandatory requirement for pharmaceutical companies to submit all direct-to-consumer advertising to the U.S. Food and Drug Administration for review prior to its use (see "FDA DTC Hearings Day 2"). AstraZeneca's original proposal suggests several tradeoffs desired by the industry. For example, AZ proposed that any pre-approved ad should be exempt from a subsequent finding by the FDA that the advertisement is misleading or inaccurate.
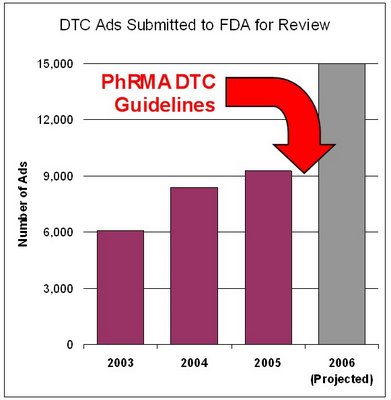 I estimated 15,000 DTC ads based on the fact that "the agency received 39,153 pieces of professional promotion for review in 2005," according to MM&M. At least half as many DTC pieces as professional pieces must be produced by the drug industry.
I estimated 15,000 DTC ads based on the fact that "the agency received 39,153 pieces of professional promotion for review in 2005," according to MM&M. At least half as many DTC pieces as professional pieces must be produced by the drug industry.[BTW, 39,153 different and unique professional promotional pieces is a remarkable achievement if you consider the 80/20 rule of promotion, which states that 80% of your effort should be focused on the top 20% of prescribers. The AMA doctor finder has 690,000 physicians in its database. 20% of that number is about 140,000, which means that practically every 3-4 doctors may get their own unique promotional piece designed especially for them! The drug industry in other words is achieving near one-to-one marketing as far as physicians are concerned!]The MM&M NewsBrief ends with this:
The addition of the second DTC review team, first reported in The Pink Sheet, has fueled discussion between the agency and industry of a possible increase in PDUFA user fees. DDMAC also maintains four review groups dedicated to professional advertising.While a recent Institute of Medicine (IOM) report was critical of the FDA and called for changes in DTC advertising (see "IOM Report Calls for DTC Moratorium"), it does not support increasing User Fees to cover FDA costs. In fact, the IOM suggests that User Fees be scaled back and that the agency get more direct funding form the government.
What's next? Mandatory Review of Professional Advertising?
"DDMAC also maintains four review groups dedicated to professional advertising," reports MM&M. "The agency received 39,153 pieces of professional promotion for review in 2005."That's about 10,000 pieces per review group or 3,000 pieces per reviewer (about the same number as the DTC reviewers would have to process). To review all these pieces, a reviewer would have to process about 15 pieces per work day.
I don't know how realistic this is. It sounds like too heavy a workload to me. There are sure to be mistakes made. IMHO, therefore, the FDA should not cave in to industry demands that a pre-approved ad be exempt from a subsequent finding by the FDA that the advertisement is misleading or inaccurate.

It is quite clear that the ratio of ads to staff leads to insufficient review. Expect more misleading ads. Research on the accuracy of drug ads has been clear on this point. Look up Loke, Cooper & Schriger, or Villanueva for examples pertaining to poor accuracy of journal ads -- I am less familiar with research addressing DTC ad accuracy, but I don't know why they would be any more accurate than ads targeted to docs.
ReplyDelete