The share of pharma spending on print ads in magazines in the first half of 2006 is up sharply, according to a TNS Media Intelligence study cited in a report by MM&M. Magazines grabbed 34% of total pharma company DTC ad spending during the first half of 2006, up from 29% in the same period in 2005. In comparison, the share allocated to TV DTC decreased from 64% to 59%.
Some analysts contend that print DTC is enjoying a windfall due to the PhRMA guidelines, which became effective in January, 2006.
"Following the adoption of PhRMA'’s voluntary guidelines earlier this year, most companies pledged to improve accuracy and balance in ads. Since this is easier to accomplish in magazine ads, drug makers are using that medium more frequently, TNS research director Jon Swallen told the Associated Press." (MM&M story)Principle 11 (Balanced Representation of Benefits and Risks) of the PhRMA Guidelines is the relevant principle here.
"DTC television and print advertising should be designed to achieve a balanced presentation of both the benefits and the risks associated with the advertised prescription medicine. Specifically, risks and safety information in DTC television advertising should be presented in clear, understandable language, without distraction from the content, and in a manner that supports the responsible dialogue between patients and health care professionals."You can see that this principle is focused almost exclusively on TV and, at best, is ambivalent with regard to print. Swallen's point may be that drug advertisers are unsure how to apply this principle to TV ads and are scaling back on TV DTC ads until they can figure it out. Meanwhile, that money has to go somewhere, therefore print ad spending is up.
Following the money, we may need to pay more attention to print DTC than we have in the past. For example, how "balanced" are print DTC ads? If it's easier to achieve balance in print than in TV ads, then we should see more balance in print, right?
One way to look at this is to measure how much time in TV ads and space in print ads is devoted to risk information.
I have looked at over 60 print ads and measured the ad space is devoted to risk information vs. benefit information in these ads. To get access to the complete dataset, please download the entire September issue of Pharma Marketing News (FREE!).
I haven't done a study of TV DTC ads, but from what I see and hear, perhaps 20% to 30% (12 to 18 seconds in a 60-second spot) of such ads are devoted to presentation of major risk (fair balance) information.
How do print ads stack up against that?
In my study, print ads I studied that appeared in publications from June through October, 2006, devote only an average of 12% of the creative ad space (not including the "brief summary" page) to risk information.
On that basis, print ads devote even less attention to risk information that do TV ads! Note, however, that I am talking only about the creative area of the ad, not the "brief summary," which is often the entire drug label that is reproduced on the opposite side of the ad page. Nobody reads this, so I don't count it.
[About half of the print ads I looked at presented a patient-friendly brief summary page. This Q&A large format style was pioneered by Pfizer. The problem is that even this friendly version is not likely to be read because it appears on the reverse side of the ad page.]In print ads appearing in publications from February through March, 2004, about 9% of the creative ad space was devoted to risk information.
This tells me two things:
- Print DTC ads devote considerably less ad space to presenting risk information than does TV DTC, and
- Print DTC ads appearing in publications after the PhRMA DTC principles became effective devote more space to risk information (about 33% more) than ads published before the principles became effective.
Do print DTC ads provide enough risk information? It depends. Some brands have more risk potential or potential for more serious risks than other brands and therefore require more space to explain the risks. Some brands, like Botox, are rogues and run only reminder print ads without any risk information at all even though the risks may be devastating.
Among the Top Ten "DTC Spending Champs" in the first half of 2006, here's the space they devote to risk information in their print DTC ads (sorry, I don't have data for Ambien/AmbienCR):
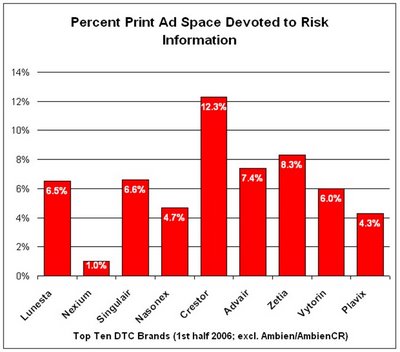 Except for Crestor, all of these top advertised brands devote less than average ad space to risk information in their print ads.
Except for Crestor, all of these top advertised brands devote less than average ad space to risk information in their print ads.Of course, it's not just the amount of information that's important, but the quality of that information. These days, there is a lot of lip service being paid to how to improve the quality of risk information presented in DTC ads. Yet it is very difficult to define "quality." The FDA is "studying" the issue.
I just don't believe that presenting "less" risk information in DTC ads as some critics have proposed (see "DTC Without the Risk") will lead to better quality information.

Interesting research, John. Extending your thinking to online pharma ads, I skimmed a few from my archive.
ReplyDeleteConsider, first, these Viagra ads from Pfizer:
http://adverlicio.us/topic/viagra
Depending on the size of the ad unit, the safety information as a % of total space ranges from ~8% to ~18%. Importantly, the safety information is persistent (always accessible to the consumer).
However, the issue's not black and white. Consider how GSK treats safety information in these Valtrex online ads:
http://adverlicio.us/industry/valtrex
By using a rollover trigger, they enable a much larger display of safety information. However, it relies on the consumer mousing over the trigger at the bottim of the ad unit. If they fail to do that, little/no risk information will have been communicated.
Lots more online pharma ads here for anyone interested in seeing how other brands are balancing risks and benefits --
http://adverlicio.us/pharma
The whole issue of risk communication seems ripe for some serious research that goes beyond measuring "inputs" (e.g. space or time in an ad) and instead actually gauges "outputs" (e.g. consumer perceptions and changes in their beliefs).
Do we have any research experts who could describe a methodology for doing this?
A study published this week shows that online advertising is a doing a terrible job at making drug information comprehensible. The amount of "space" (online, broadcast, or print) devoted to risk info is one key factor, but another is the understandability of the information. See below. This is worth a blog entry in itself
ReplyDeleteFrom FDANews.com, 10/13:
DRUG WEBSITE LANGUAGE, DESIGN HINDER UNDERSTANDING OF DRUGS, STUDY SHOWS
Close to half of the U.S. population does not fully understand the information on drug websites that target consumers, according to an analysis — further evidence of "a serious, serious problem" in consumers' understanding of medicines and how to take them, according to one consumer advocate.
Understanding content on the average drug website requires 12 years of education, meaning that only 55 percent of the U.S. population can fully understand the information provided on these sites, the analysis by healthcare marketing firm Campbell-Ewald Health found. More than three-quarters of the websites do not offer information in Spanish, and only half were designed using standard guidelines to improve the usability and accessibility of websites, the study showed.
"This is not news," Bill Vaughan, senior policy analyst with Consumers Union, said. "The American public are not doctors and have trouble understanding [drug information], and doctors have trouble conveying it, and we all need to work together to do a better job."
The analysis rated 58 drug websites across nine therapeutic categories based on several qualities, including website design, ease of accessing and absorbing the content, whether there is a good overview of the condition the drug treats and how easy it is for consumers to look up signs and symptoms of the disease. The analysis also considers how well an average consumer can understand — based on the information included on a website — how a drug works, what to expect when taking the drug and whether warning signs are explained clearly and simply. Other factors the researchers took into account was how well a website accommodates people looking up information for another person, and whether consumers can access research studies and clinical trial data.
The websites that scored the highest, including Byetta.com, Lipitor.com and Seroquel.com, are "clear, easily understood and engaging sites," the study said. "Users can navigate easily, and it is simple to determine where the user is within the site."